Sponsored Content:
This content is not an endorsement from the ABTA.
Sponsored by GT Medical Technologies, Inc.
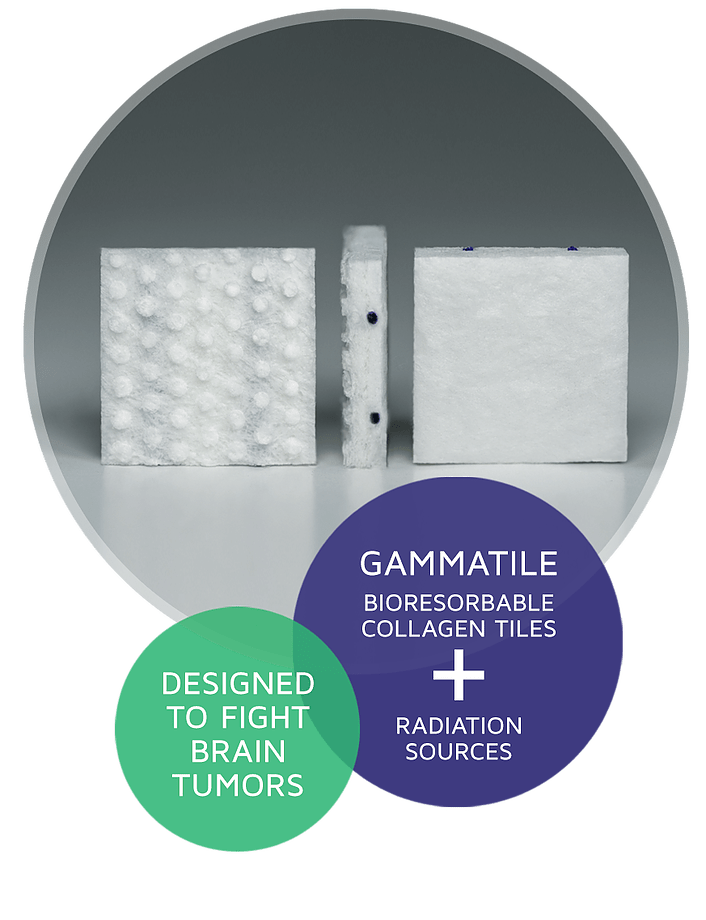
A radiation treatment specifically designed for use inside the brain, GammaTile Therapy gives patients new hope in the fight against brain tumors. GammaTile Therapy is a safe and effective Surgically Targeted Radiation Therapy (STaRT) for operable brain tumors that begins working immediately at the time of brain tumor removal.1
GammaTile Therapy can eliminate the need for traditional repeat radiation treatments and associated hospital or clinic visits, so patients can focus on what matters most—healing and getting back to their daily lives sooner. GammaTile is the most recent FDA-cleared medical device to treat brain tumors, and is covered by Medicare and most private insurance companies.
Learn more about GammaTile Therapy.
Tough on the Tumor. Easier on Patients and Caregivers.
- One and done radiation treatment: patients receive their radiation while going about their daily lives
- Delays brain tumor recurrence, potentially extending survival1
- Collagen tile protects healthy brain tissue from radiation exposure, reducing the risk of radiation-related side effects and preventing hair loss2
- FDA-cleared for newly diagnosed malignant tumors and recurrent brain tumors, including brain metastases, gliomas, and meningiomas

Physician Perspective
“We are excited to offer this new treatment that protects healthy brain tissue while providing immediate, targeted therapy directly to the area that’s most at risk for recurrence. Most patients report fewer side effects and better quality of life since it eliminates the wait time and multiple visits typically associated with standard radiation treatment after the removal of a tumor.”
Dr. Julian Bailes
Neurosurgeon at NorthShore University Health System
GammaTile is being offered at top Brain Tumor Centers across the United States. Find a GammaTile treatment center near you.
Patient Perspective
“After I had the GammaTile procedure, I felt fine—as if it didn’t happen. The recovery was great. I was back in the gym quickly and able to bounce back and be me again.
GammaTile actually gave me a higher sense of security because it’s well known that surgery alone doesn’t…take out all the [tumor] cells. You need something…to knock out whatever is left. Knowing that I had GammaTile felt comforting”
OSCAR | HIGH-GRADE GLIOMA SURVIVOR

Learn more about Oscar’s journey.
Important Safety Information:
GammaTile Therapy is intended to deliver radiation therapy in patients with newly diagnosed malignant brain tumors and recurrent brain tumors. The potential for, and symptoms of, adverse events related to radiation exposure vary depending on the radiosensitivity of the exposed tissue, the amount of radiation delivered, and the placement of GammaTile(s). Serious side effects related to GammaTile Therapy are rare, and may include radiation brain changes including necrosis. GammaTile Therapy should not be used for patients with known history of hypersensitivity to bovine-derived materials. Possible complications can occur with any neurosurgical procedure, including cerebrospinal fluid leaks, infection, delayed hemorrhage, and adhesion formation.
References
- Nakaji P, Youssef E, Dardis C, Smith K, Pinnaduwage D, Brachman D. Surgically targeted radiation therapy: a prospective trial in 79 recurrent, previously irradiated intracranial neoplasms. Poster presented at: 2019 AANS Annual Scientific Meeting; April 2019; San Diego, CA.
- Brachman D, Youssef E, Dardis C, Smith K, Pinnaduwage D, Nakaji P. Surgically targeted radiation therapy: Safety profile of collagen tile brachytherapy in 79 recurrent, previously irradiated intracranial neoplasms on a prospective clinical trial. Brachytherapy. 2019;18(3):S35-S36.
©2020 GT Medical Technologies, Inc.