**Sponsored by Boston Scientific: This content is not an endorsement from the ABTA.**
Boston Scientific transforms lives through innovative medical technologies that improve the health of patients around the world. As a global medical technology leader for more than 40 years, we advance science for life by providing a broad range of high-performance solutions that address unmet patient needs. Our portfolio of devices and therapies helps physicians diagnose and treat complex cardiovascular, respiratory, digestive, oncological, neurological and urological diseases and conditions.
TheraSphere™ Y-90 Glass Microspheres have been used for over 20 years to treat hepatocellular carcinoma (liver cancer) and over 100,000 patients worldwide. The purpose of the FRONTIER study is to determine the ability to safely use the Y-90 technology for the treatment of recurrent glioblastoma.
Recurrent Glioblastoma
Glioblastoma is one of the most aggressive forms of brain cancer, and unfortunately, it often recurs even after initial treatments. Yet, amidst this challenge, researchers and healthcare professionals persist in seeking novel therapies to improve outcomes and enhance quality of life for those affected by this disease. One novel treatment under investigation is Yttrium-90 (Y-90) glass microspheres for recurrent glioblastoma.
How Y-90 glass microspheres work
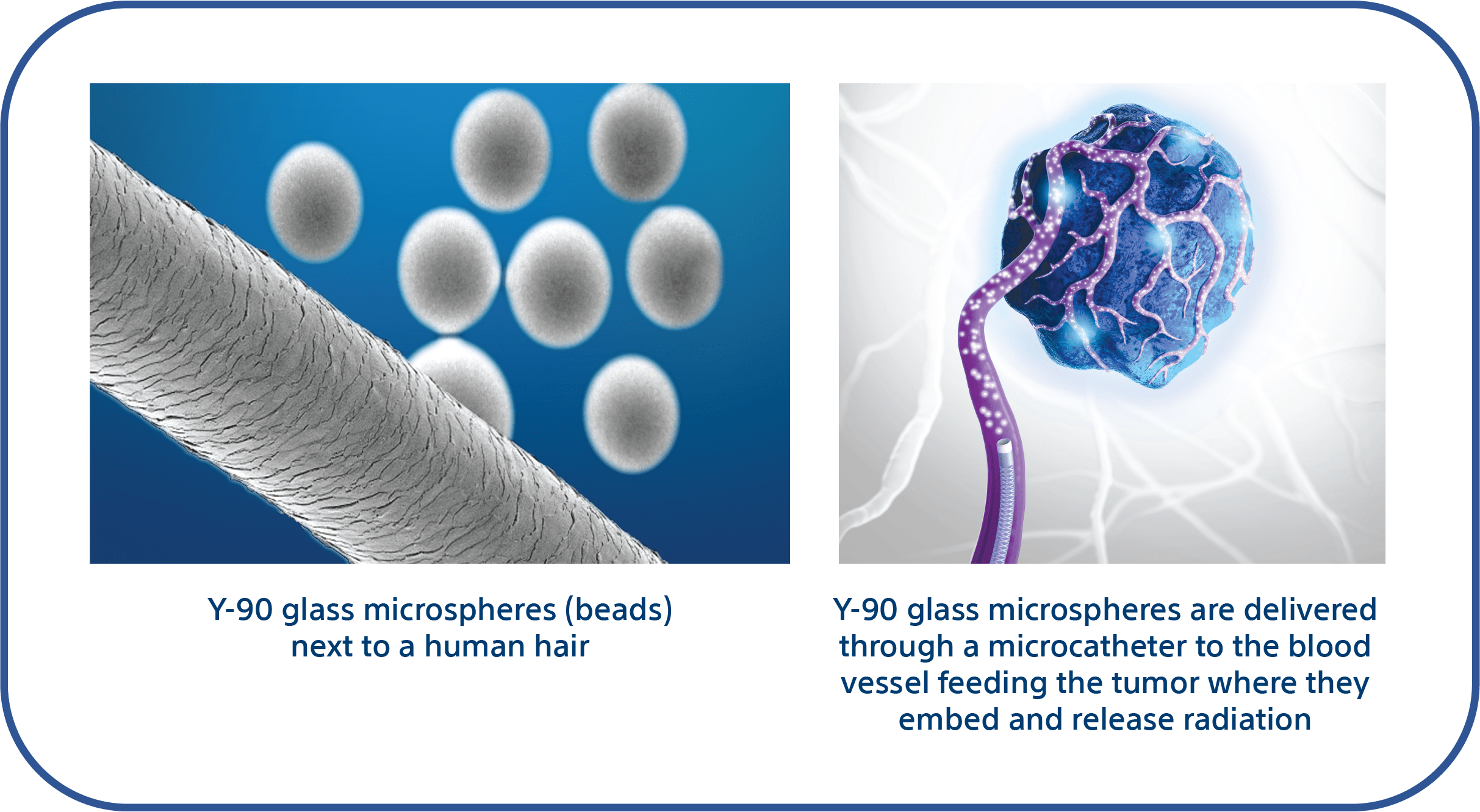
Y-90 for recurrent glioblastoma is an investigational internal radiation treatment with the goal of directly targeting the cancerous tumor while minimizing harm to surrounding healthy tissue. This minimally invasive approach involves delivering radioactive microscopic glass beads, via a small tube called a microcatheter, directly into the blood vessels feeding the tumor. The Y-90 glass beads embed within micro blood vessels and release radiation over the following weeks.
Unlike traditional radiation therapy, which delivers radiation from an external source, Y-90 therapy delivers radiation internally at the tumor site. For further explanation, watch this short animated video via PRIMR:
FRONTIER study
In the FRONTIER study (NCT05303467), participants will receive a single Y-90 treatment and be monitored over the course of the study to see how they respond. The study aims to evaluate the safety of using Y-90 in the brain. Additionally, the study will evaluate the accuracy and effectiveness of this therapy for recurrent glioblastoma.
Study enrollment is underway, with up to 12 patients being recruited across five active sites in the United States.
More information
If you or a loved one is facing recurrent glioblastoma, talk to your doctor to see if you might be eligible to join the trial or if there are other treatment options available to you. With the dedication of researchers, clinicians, and the bravery of patients participating in trials, we can continue to make progress in the fight against this devastating disease.
For additional information on the study, patient brochure and enrolling sites, please visit FRONTIER study – Boston Scientific.

CAUTION: Investigational Device. Limited by Federal (or US) law to investigational use only. Not available for sale.
PI-1836102-AA

Jessie Schlacks
Jessie is Managing Editor of the bi-monthly e-newsletter MindMatters. Submit story ideas or questions to jschlacks@abta.org.