Sponsored by Imvax: This content is not an endorsement from the ABTA.

At Imvax, we are united behind advancing a new approach to personalized, whole tumor-derived immunotherapy that targets the treatment of intractable solid tumors.
Imvax is a clinical-stage biotechnology company headquartered in Philadelphia, PA, with a unique platform technology, Goldspire™. Goldspire harnesses decades of research and multiple validated technologies by seeking to capture the full antigen signature of a patient’s tumor and using it to stimulate the patient’s own innate and adaptive immunity against tumor cells. Goldspire is designed to fit seamlessly into existing standard of care (SOC) therapy.
(Click image to view larger version)
How it works
Our lead asset, IGV-001, is an autologous biologic-device combination product that involves a unique approach to inducing a broad and durable immune response against tumors. After surgical removal and transfer to Imvax’s state-of-the-art manufacturing facility, patient tumor samples undergo rapid tissue processing (i.e., less than 24 hours). Imvax incubates a patient’s tumor cells with antisense and then places the cells into proprietary, implantable biodiffusion chambers (BDCs). These are irradiated with a low dose of radiation before being returned to the hospital for implantation in the patient for approximately 48 hours.
Data from Imvax’s preclinical studies suggest that the antigens and other immunostimulatory factors released from the BDCs stimulate nearby immune cells. These cells then travel to local lymph nodes to induce long-term adaptive immune responses against remaining tumor tissue in the body.
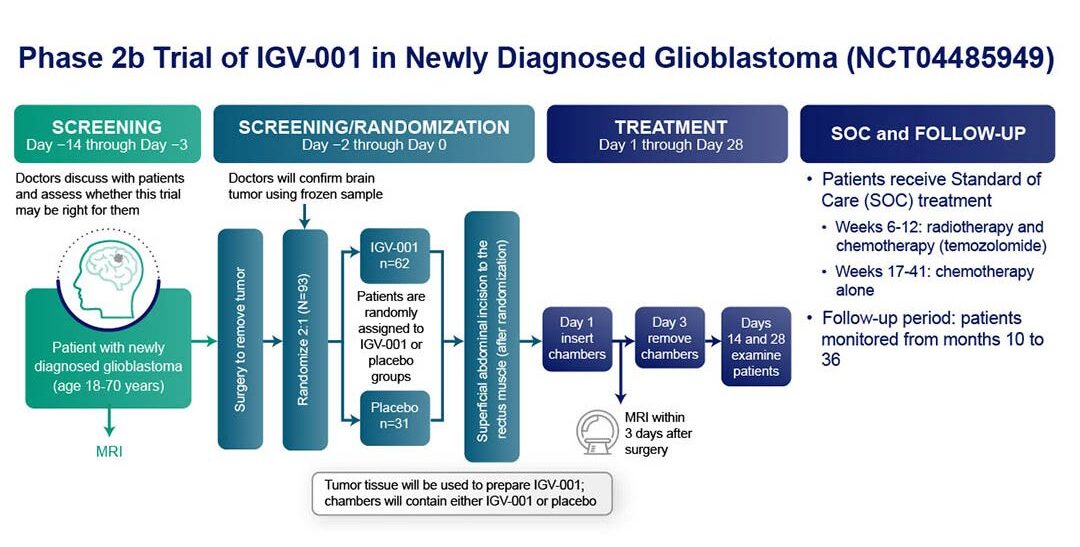
About our study
We recently initiated a randomized, multicenter, double-blind, placebo-controlled, Phase 2b trial (NCT04485949) to assess IGV-001 in newly diagnosed patients with glioblastoma (ndGBM) post-craniotomy. This study compares the efficacy of a one-time treatment with IGV-001 plus SOC GBM therapy (radiotherapy + temozolomide) versus placebo plus SOC GBM therapy alone.
The study will enroll 93 patients randomized 2:1 (IGV-001 + SOC vs SOC) at up to 25 US sites. The primary efficacy endpoint of the trial is progression free survival (PFS) and the secondary efficacy endpoint is overall survival (OS). Safety and tolerability of IGV-001 are the safety objectives of the study. You can find additional information about the ongoing Phase 2b trial in the Patients & Families page of our website.
Imvax’s IGV-001 glioblastoma program seeks to build on groundbreaking Phase 1b data that showed that IGV-001 was safe and well tolerated. A Phase 1b study yielded several efficacy signals, including significant improvements in progression free survival (PFS), overall survival (OS), radiographic evidence of tumor response, and multiple biomarker changes that supported the presence of an immune response (Andrews DW, et al., Clin Cancer Res. 2021;27(7):1912-1922).
In ten Stupp-eligible ndGBM patients in the highest dose cohort treated with IGV-001, median PFS was 17.1 months, compared with 6.5 months in historical SOC treatment, and median OS was 38.2 months, compared with 16.2 months in historical SOC. For additional information, please visit www.imvax.com.

Jessie Schlacks
Jessie is Managing Editor of the bi-monthly e-newsletter MindMatters. Submit story ideas or questions to jschlacks@abta.org.