From groundbreaking research to new collaborations at a federal level, discover the top three brain tumor news highlights and milestones for 2023:

First Targeted Therapy for Low-grade Glioma Heads for FDA Approval
This summer, the New England Journal of Medicine published the groundbreaking results of a novel targeted therapy for low-grade gliomas with IDH1 or IDH2 mutations.
The phase 3 trial studied an oral drug called vorasidenib, which is capable of bypassing the blood-brain barrier to hinder tumor growth without being toxic to other areas of the brain.
Study results found that patients who received the drug went more than twice as long before experiencing tumor progression compared to the placebo group (approx. 27.7 months versus 11.1). In turn, about 83 patients who received the drug did not show a need for additional treatment intervention at the two-year mark.
Earlier this year, the FDA granted fast-track designation to the manufacturer, Servier, to produce more of the drug.
Pending FDA approval, the drug could become widely available to patients as soon as next year.
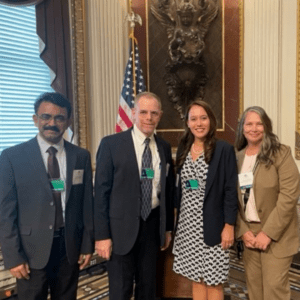
White House Hosts Cancer Moonshot Brain Cancers Forum
In May, neuro-oncology leaders from across the country gathered in DC to discuss opportunities in discovering treatments for brain cancers, including GBM—the deadliest form of brain cancer. The White House Cancer Moonshot Initiative, which was reignited by the Biden Administration in 2022, aims to cut the cancer death rate in half by 2047.
ABTA Chief Mission Officer Nicole Willmarth, PhD, joined the Brain Cancers Forum and noted the renewed interest and urgency to drive progress quickly. Some issues of focus included: the need to better understand tumor heterogeneity (differences between cancer cells within a single tumor): overcoming drug delivery challenges with the blood-brain barrier; and finding models from other cancer treatments that could be applied to brain cancer.
Willmarth said we’re on the cusp of different discoveries that could offer major improvements to patients in the next five to 10 years. She adds that the collaborative effort across disciplines and the individual commitments made by advocacy groups are key to achieving Cancer Moonshot goals.
ABTA Raises the Bar for Patient Support Services and Research
As the ABTA marked its 50th year of service this year, it saw significant growth in its patient support services and research grants program.
In 2023, the ABTA:
- Served more than 100,000 patients and caregivers through its programs and services
- Hosted its National Conference in person and online with a record-breaking 1,400 attendees
- Exceeded 1,000 patient and caregiver matches through its Patient & Caregiver Mentor Support Program
- Funded $1.3 million in research grants and fellowships (the most funded since 2012!)
To further accelerate research discoveries and improve the lives of patients, the ABTA also launched its five-year, $50 million Meet Hope Head On campaign in March. The goal: triple the ABTA’s research investment, double the number of patients served, and double federal funding for brain tumor research.
Learn more about Meet Hope Head On and how you can get involved.

Jessie Schlacks
Jessie is Managing Editor of the bi-monthly e-newsletter MindMatters. Submit story ideas or questions to jschlacks@abta.org.